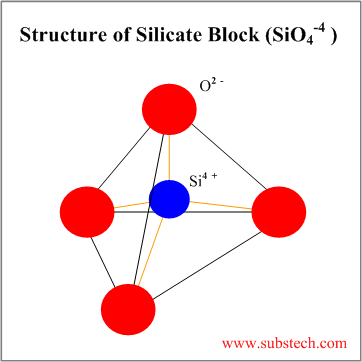
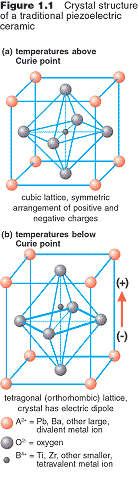
Types Of Ceramic Crystal Structures

Structures of ceramics outline introduction crystal structures ceramic structure ax type crystal structures amxp type ambnxp type silicate ceramics carbon ceramic structures two or more different elements more complex than metal structures ionic and or covalent bonds a mix of ionic and covalent bonds.
Types of ceramic crystal structures. However it should be noted that the crystal structures of ceramics are many and varied and this results in a very wide range of properties. Ceramic crystalline or partially crystalline material most ceramics usually contain both metallic and nonmetallic elements with ionic or covalent bonds. This is analogous to ferromagnetism in that in the absence of an electric field during production the ferroelectric crystal does not exhibit a polarization. The structure of most ceramics varies from relatively simple to very complex.
Each collection of ions is shown in an overall box that describes the unit cell of that structure. Therefore the structure the metallic atoms the structure of the nonmetallic atoms and the balance of charges produced by the valence electrons must be considered. Electronegativity is the capability of the nucleus in an atom to attract and retain all the electrons within the atom itself and depends on the number of electrons and the distance of the electrons in the outer shells from the nucleus. In the latter case the glassy phase usually surrounds small crystals bonding them together.
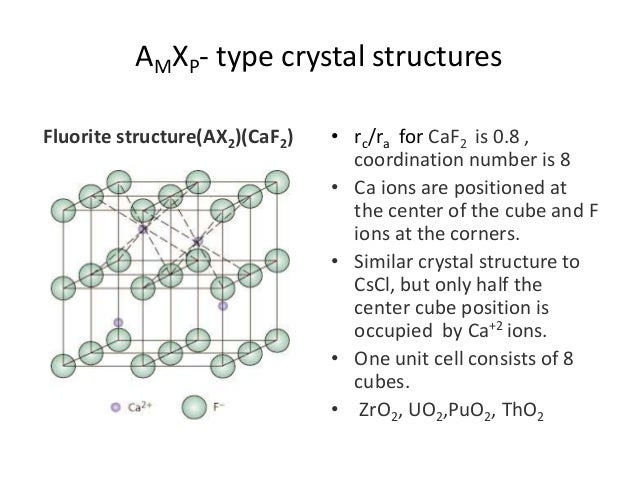
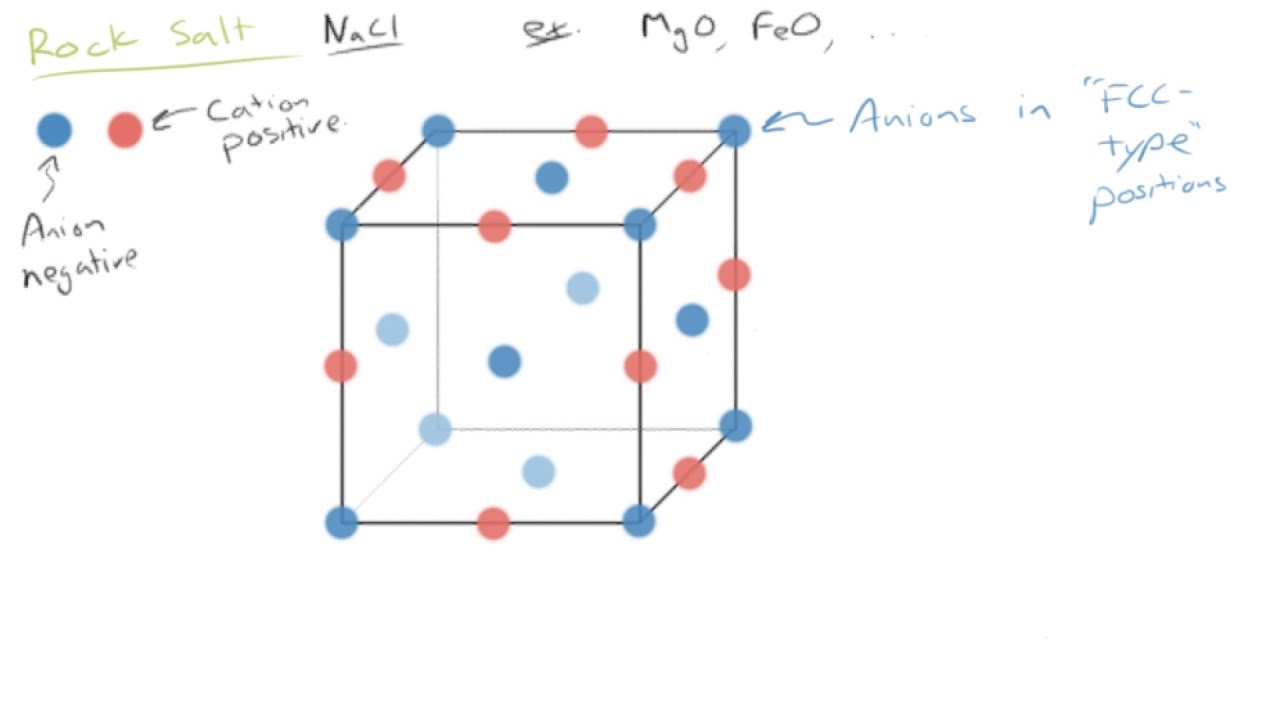
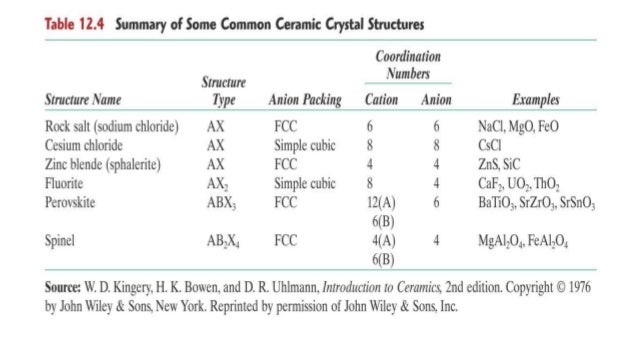
Crystal structure is also responsible for many of the properties of ceramics. Upon the application of an electric field of sufficient magnitude the crystal becomes permanently polarized. In figures 2a through 2d representative crystal structures are shown that illustrate many of the unique features of ceramic materials. Common examples are earthenware porcelain and brick.
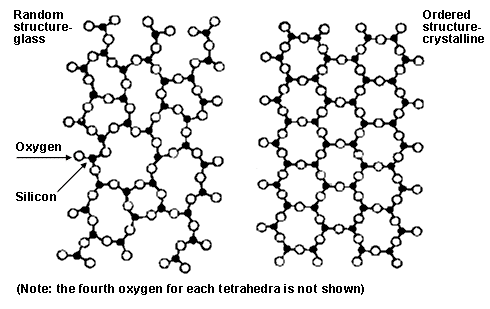
The microstructure can be entirely glassy glasses only. The ionic bond occurs between a metal and a nonmetal in other words two elements with very different electronegativity. The crystallinity of ceramic materials ranges from highly oriented to semi crystalline vitrified and often completely amorphous. A ceramic is any of the various hard brittle heat resistant and corrosion resistant materials made by shaping and then firing a nonmetallic mineral such as clay at a high temperature.
Most often fired ceramics are either vitrified or semi vitrified as is the case with earthenware stoneware and porcelain. Two types of bonds are found in ceramics. There are a few crystal structures notably the perovskite structure which exhibit ferroelectric behavior.